Leading the field in molecular diagnostics
Our Testing and Passporting System (TAPS)
Begin your testing with Clifton Life Science's COVID-19 testing and monitoring solution.
Clifton Life Sciences Testing and Passporting System (TAPS)
Clifton Life Sciences offer a turnkey novel testing and passporting system that will help your company and staff return to work more safely.
Our system offers customers:
- Access to a cutting-edge RNA based PCR COVID-19 test with unrivalled accuracy.
- A saliva-based test that is easier and less uncomfortable to use.
- Testing frequency to be determined on a user by user basis.
- A dedicated portal under the control of a company’s human resources department ensuring that all identifiable information is kept under their control.
- A personalised app for each testing subject including their current testing status.
The TAPS smartphone app
We offer a turnkey novel testing and passporting system that will help your company and staff return to work more safely.
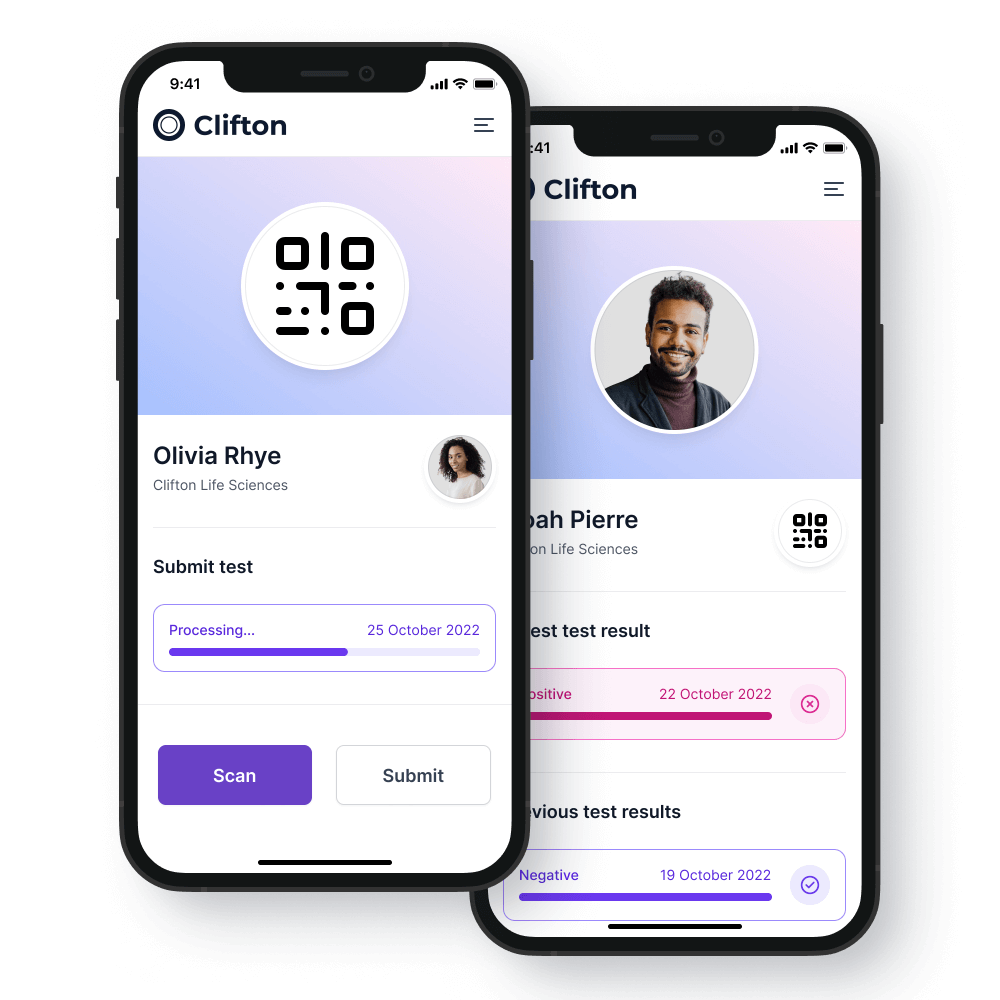
Our system offers
Access to a cutting-edge RNA based PCR COVID-19 test with unrivalled accuracy.
A saliva-based test that is easier and less uncomfortable to use.
Testing frequency to be determined on a user by user basis.
Personalised app
A dedicated portal under the control of a company’s human resources department ensuring that all identifiable information is kept under their control.
A personalised app for each testing subject including their current testing status.
The test
We have a world class non-invasive COVID-19 molecular genomic saliva test that will accurately deliver a positive or negative result in less than 24 hours.
The test was developed by Wren Laboratories www.wrenlaboratories.com a Yale University faculty spinoff that functions as a CLIA authorized laboratory and is part of the global Clifton Life Sciences group
One of the key benefits of our saliva test are that it is much easier and less uncomfortable to obtain saliva samples than nasopharyngeal swabs or blood samples which most other companies used. In addition:
- The test is highly accurate (>99%) for detecting SARS-CoV-2 RNA and highly sensitive (100%).
- Head-to-head comparisons with other assays demonstrate that the Wren saliva assay is significantly more accurate for COVID-19 detection.
- The test is also easier for employees to use since saliva sample collection is simple and significantly more comfortable than swab collection.
- The test is currently in the final stages of obtaining FDA Emergency Use Authorization.
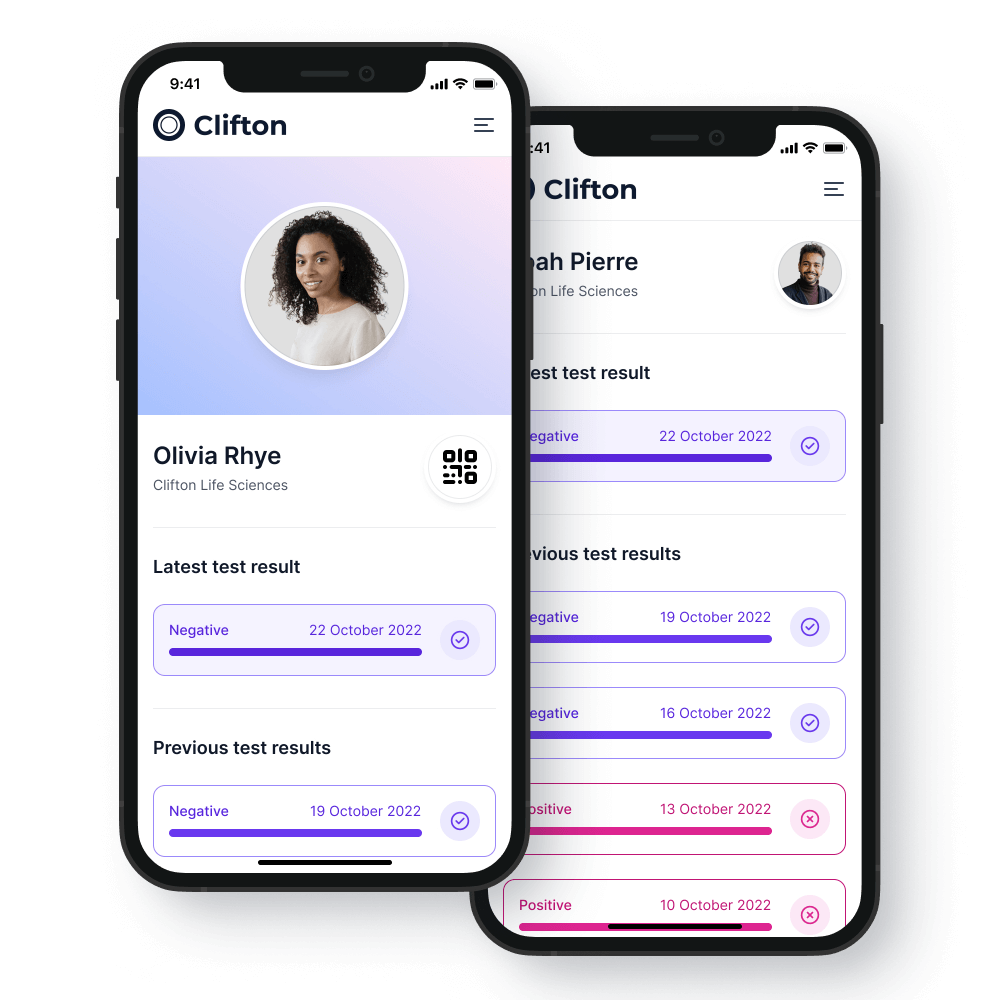
The client portal
All patient data is anonymized so that sensitive data is not exposed to malicious attack.
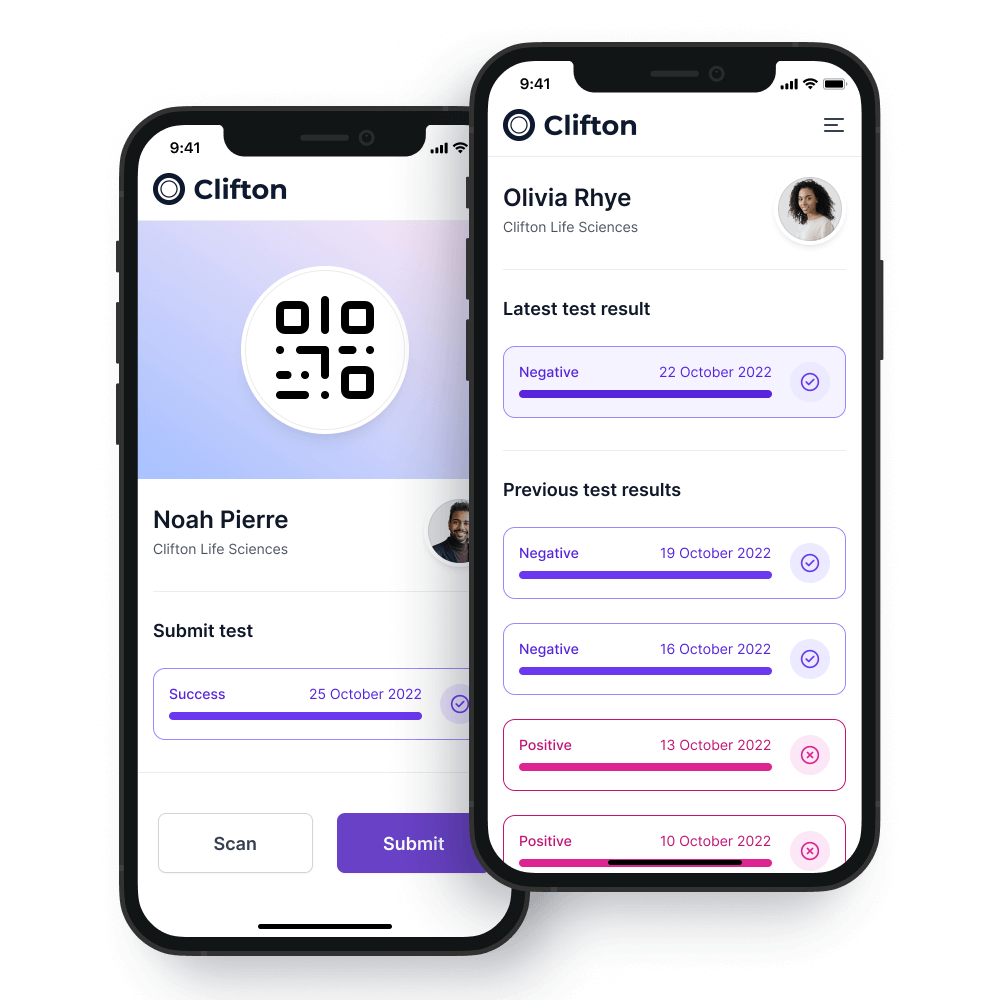
A test is initiated when a member of staff scans a test kit with their smartphone.
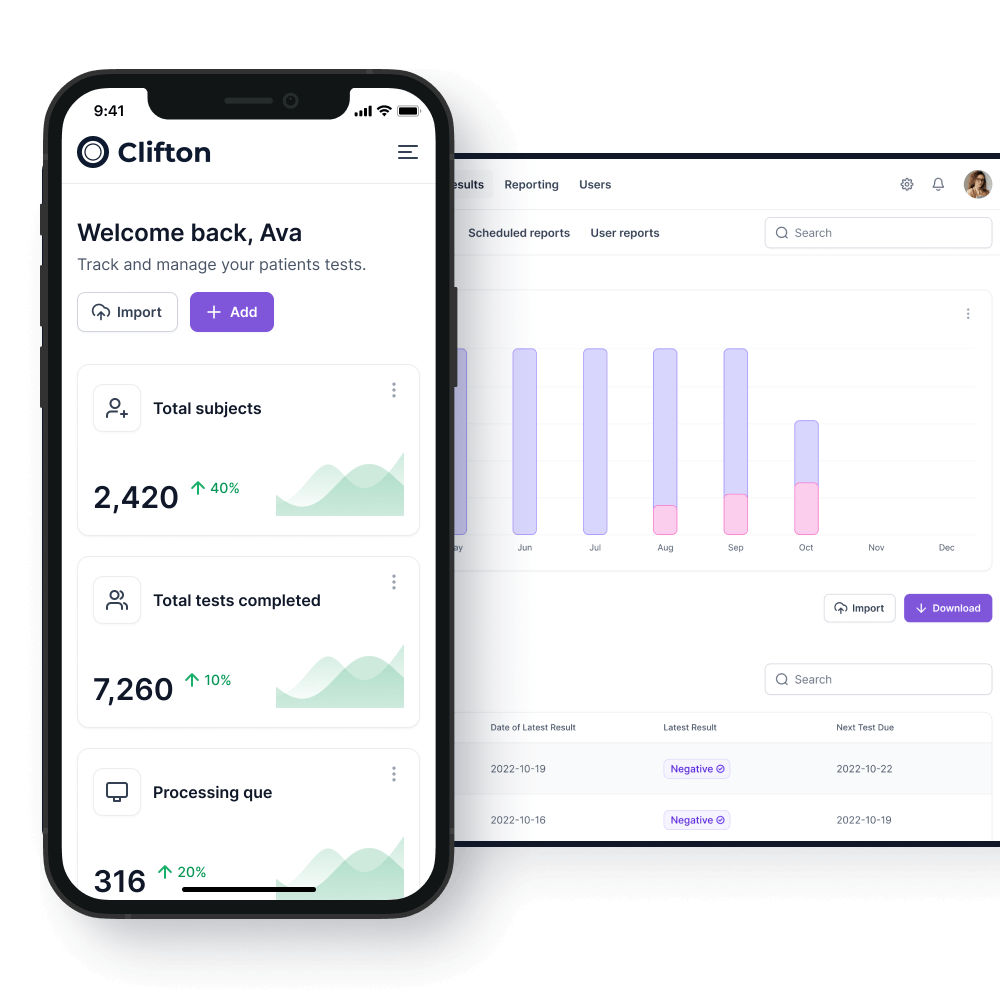
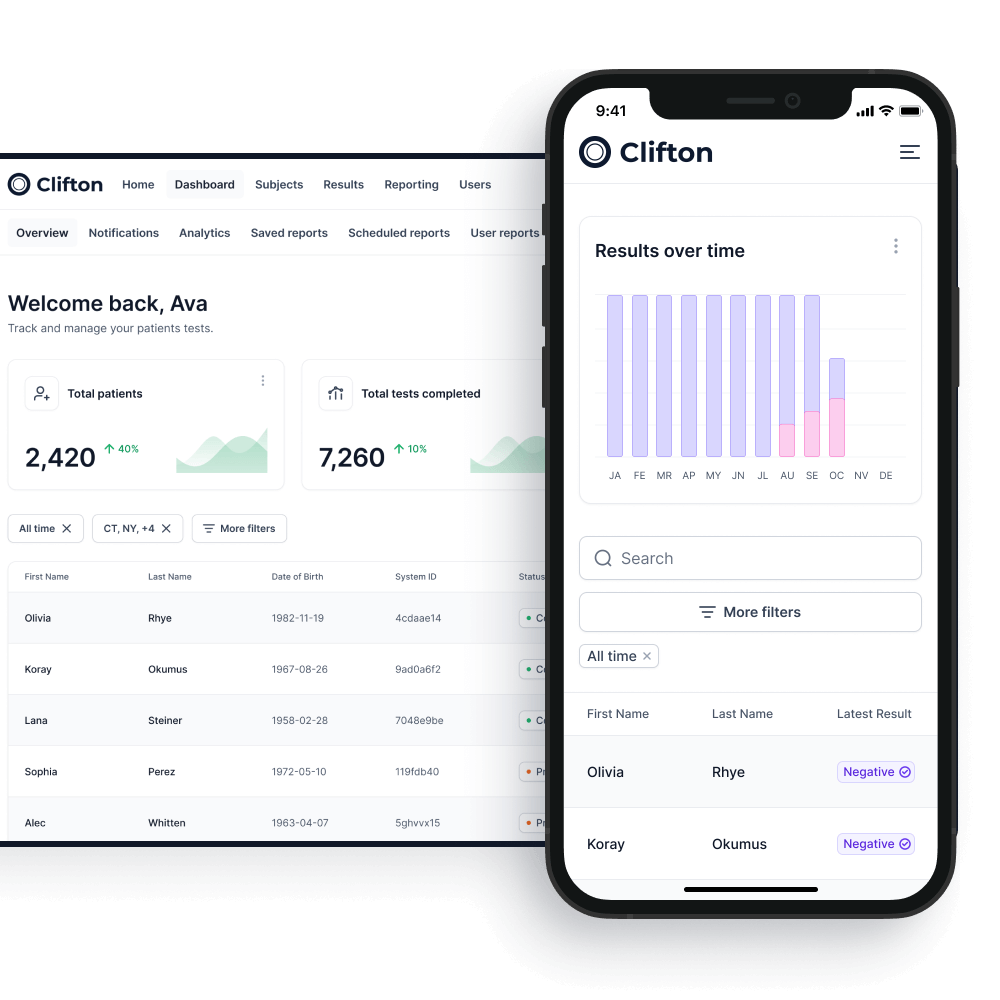
Results
The sample is then dispatched to our laboratory in the prepaid packaging.
On arrival at the laboratory the test kit is scanned again to ensure full traceability of all tests.
As the tests are processed the COVID-19 passport will be updated on both the employee smartphone and the on-premises system within your organization.
The passporting system
Our customers have identified a need to test staff regularly, obtain the results quickly and protect sensitive employee information:
- The unique Clifton passporting system allows the customer to identify testing subjects and testing frequency on an on-premises application.
- Testing subjects will be able to download an app onto their Android or iOS smartphones which will keep track of their current testing status.
- All patient data is anonymized so that sensitive data is not exposed to malicious attack.
- A test is initiated when a member of staff scans a test kit with their smartphone.
- The sample is then dispatched to a local laboratory which is authorized and trained to perform the WREN protocol.
- On arrival at the laboratory the test kit is scanned again to ensure full traceability of all tests.
- As the tests are processed the COVID-19 passport will be updated on both the employee smartphone and the on-premises system within your organization.
The passporting system
Our customers have identified the need to test their staff regularly, obtain the results quickly and protect sensitive employee information.
We have partnered with the European developed TAPS (Testing and Passporting System) to offer this service.
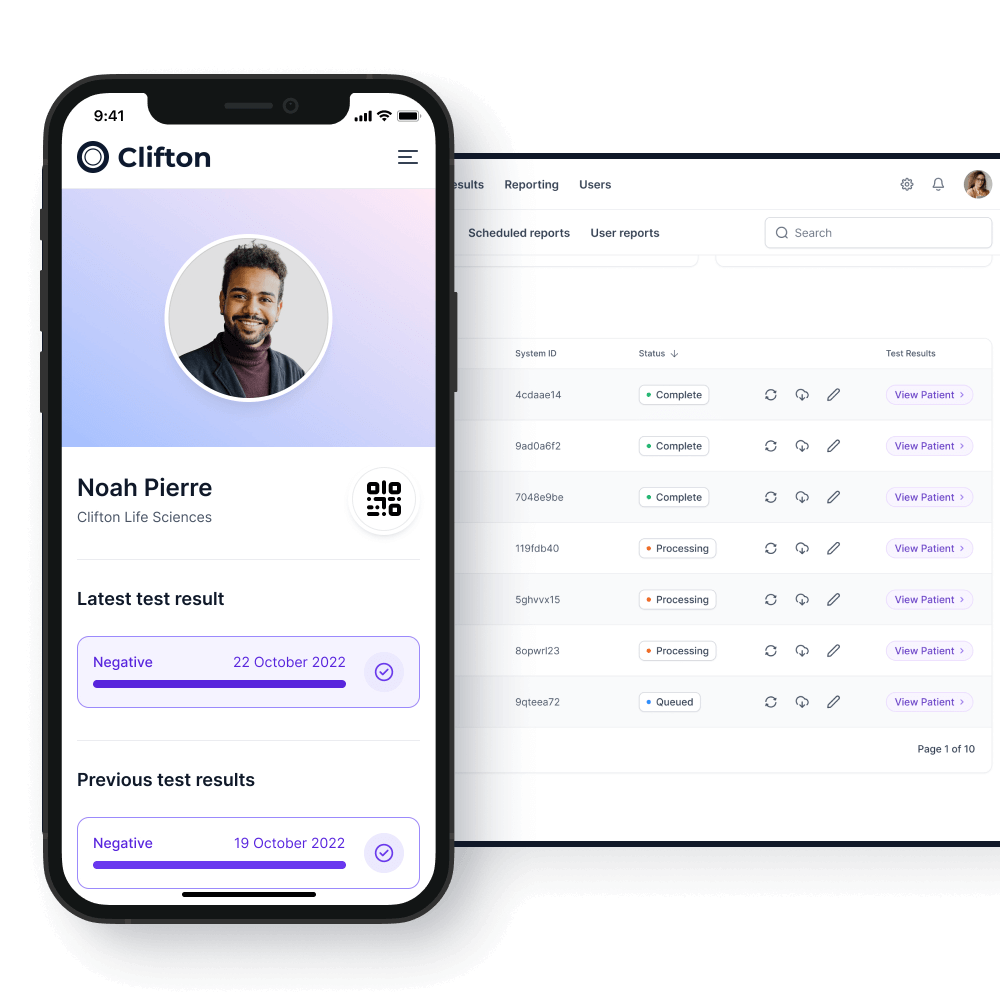
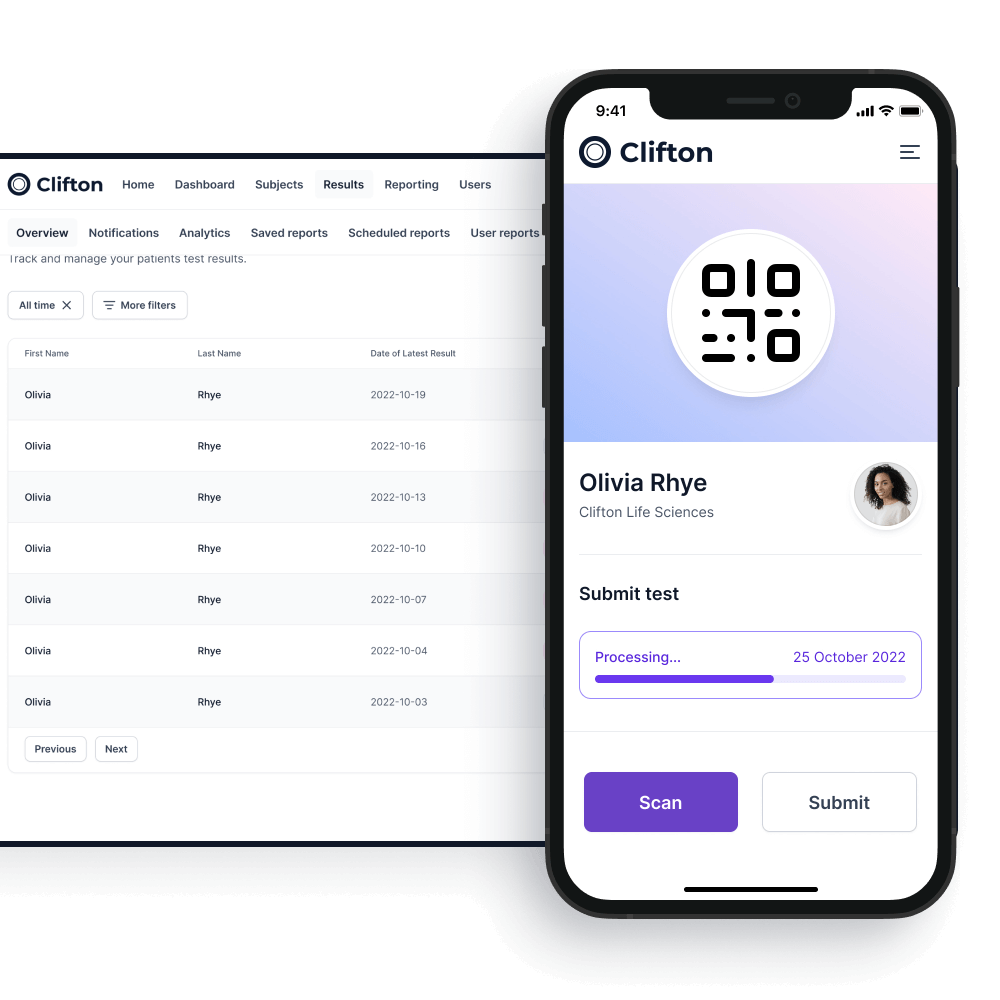
A unique system
The unique passporting system allows the customer to identify testing subjects and testing frequency on an on-premises application.
Testing subjects will be able to download an app onto their Android or iOS smartphones which will keep track of their current testing status.
Get in touch to discuss our Neuroendocrine and COVID-19 testing solutions.